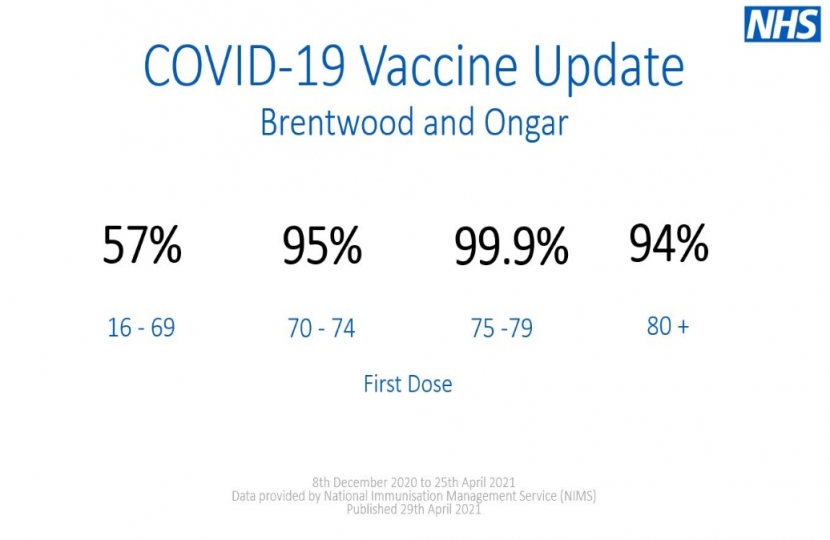
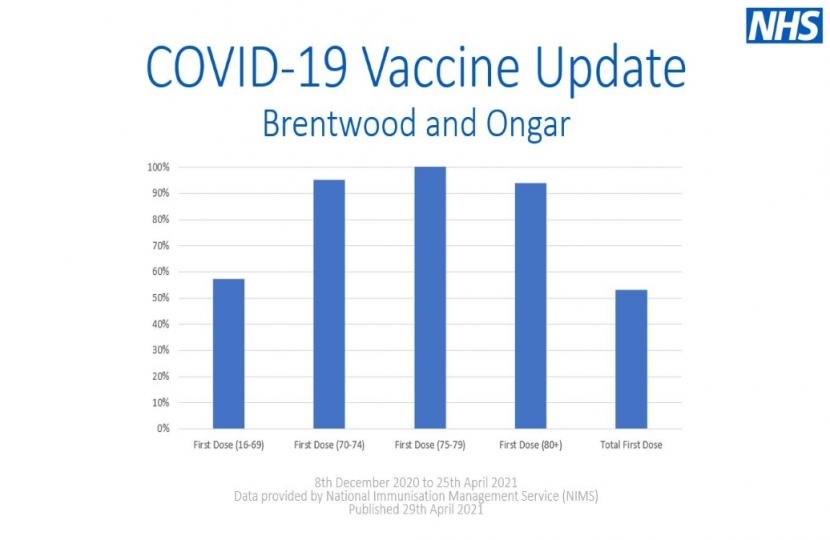
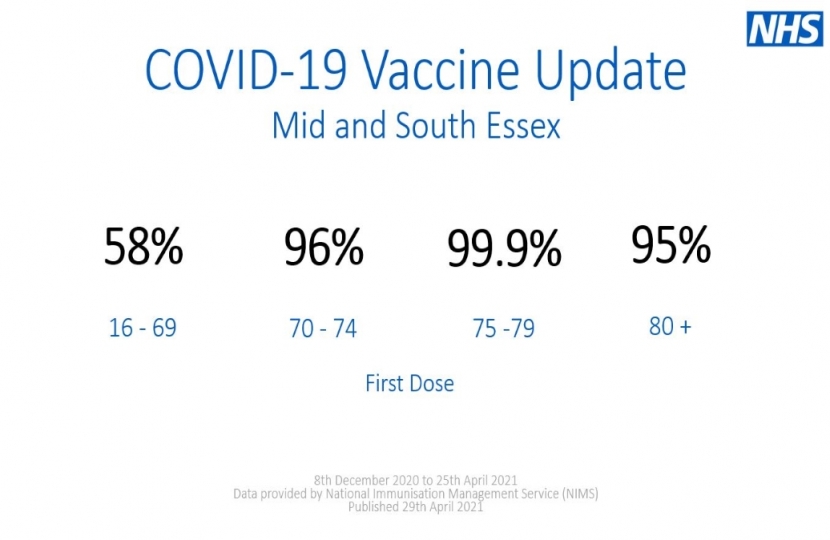
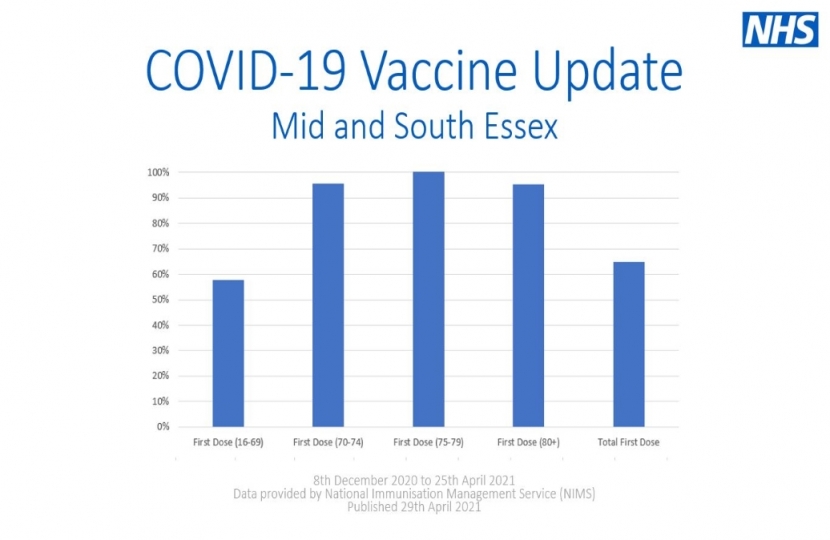
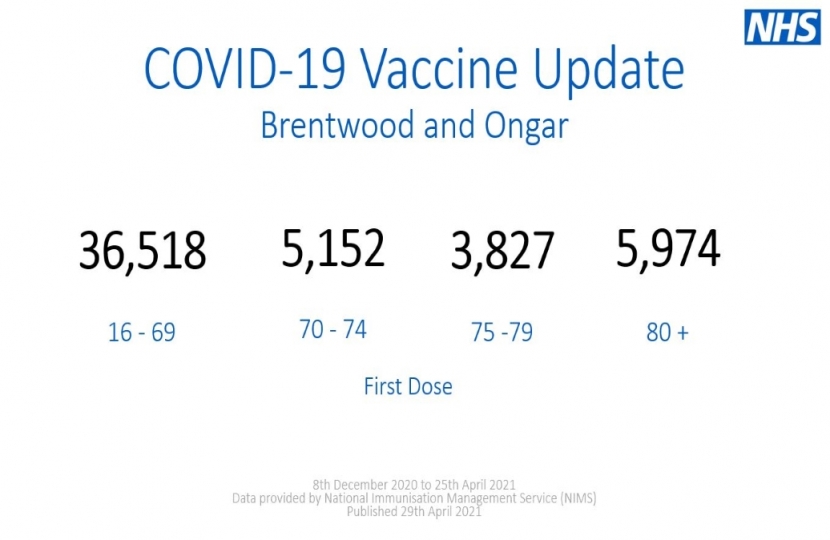
As of the 25th April, 53% of people in Brentwood and Ongar have now had at least one dose of a COVID-19 vaccine and, as a nation, we are still on target to deliver vaccines to all adults by the end of July.
On the 11th January, the Government published their Covid-19 Vaccine Delivery Plan which details how the UK government was able to build up a supply of vaccines and how it is planning to deploy them. The Plan sets out how the Government will work with the NHS, devolved administrations, local councils and the armed forces to deliver the largest vaccination programme in British history.
It is important to stress that we have a clear exit strategy, which will allow us to return to normal life. Thanks to a truly worldwide scientific effort, we are now rolling out the biggest vaccination programme in our history. With the approval our own Oxford vaccine, the pace of vaccination can increase further.
As of the 16th February: We have now offered a vaccine to everyone in the top four priority groups. In less than 10 weeks we have vaccinated 15 million people – that is one in every four adults.
The Prime Minister set out the roadmap on the 22nd February saying as much as he possibly could about the route back to normality. We want this lockdown to be the last – and we want progress to be cautious but also irreversible which is why it is so important that everyone continues to follow the rules.
On a local level, the NHS in Essex have launched a new website, giving residents the latest information and advice on the local COVID-19 vaccination programme - read more here.
LATEST: The Government met their target to vaccinate the most vulnerable by the 15th April, meaning that all those in the target Groups 1-9 have now been offered a life-saving COVID-19 jab, more than 60 million of which have been given in total: over 4 in 10 of adults are fully vaccinated. That is why, in line with JCVI recommendations to prioritise people by age, the Government are now opening up invitations to receive a vaccine to all those aged over 33.
All the latest information on the local vaccination programme can be found here - the information details the location of vaccination sites, GP vaccination sites, and details on how the vaccine is administered.
The latest official UK Government data and insights can be found here.
Further information also available here.
Additional information to further support constituents will be updated regularly here
Frequently Asked Questions
Q: Are vaccines effective against the B.1.617.2 variant first identified in India?
A new study by Public Health England shows for the first time that 2 doses of the COVID-19 vaccines are highly effective against the B.1.617.2 variant first identified in India.
More information can be found here.
Q: Why have you changed the dosage schedule?
The four UK Chief Medical Officers agree with Joint Committee for Vaccination and Immunisation (JCVI) advice that prioritising the first doses of vaccine for as many people as possible on the priority list will protect the greatest number of at-risk people overall in the shortest possible time. The decision we have taken will literally double the number of people who are protected over the next few crucial months. Everyone will still receive a second dose within 12 weeks of their first.
The Joint Committee for Vaccination and Immunisation has advised that the second dose of the Pfizer vaccine may be given between 3 to 12 weeks following the first dose, and that the second dose of the AstraZeneca (Oxford) vaccine may be given between 4 to 12 weeks following the first dose. The Medicines and Healthcare products Regulatory Agency (MHRA) also clarified that for the Pfizer/BioNTech vaccine, the interval between doses must be at least 3 weeks. For both the AstraZeneca (Oxford) and Pfizer/BioNTech vaccines, data provided to MHRA demonstrate that while efficacy is optimised when a second dose is administered, both offer considerable protection after a single dose, at least in the short term.
Q: Who gets the vaccine when?
Phase One
The Joint Committee for Vaccination and Immunisation (JCVI) advises that the first priorities for the COVID-19 vaccination programme should be the prevention of mortality and the maintenance of the health and social care systems. As the risk of mortality from COVID-19 increases with age, prioritisation is primarily based on age. The order of priority for each group in the population corresponds with data on the number of individuals who would need to be vaccinated to prevent one death, estimated from UK data obtained from March to June 2020:
- residents in a care home for older adults and their carers
- all those 80 years of age and over and frontline health and social care workers
- all those 75 years of age and over
- all those 70 years of age and over and clinically extremely vulnerable individuals
- all those 65 years of age and over
- all individuals aged 16 years to 64 years with underlying health conditions which put them at higher risk of serious disease and mortality
- all those 60 years of age and over
- all those 55 years of age and over
- all those 50 years of age and over
It is estimated that taken together, these groups represent around 99% of preventable mortality from COVID-19.
Phase Two
JCVI has recommended that the vaccine programme proceed on the basis of age groups, with those aged 40 - 49 invited first once the initial priority phase has been completed; a strategy centred specifically on occupational groups would be more complex to deliver and may require new vaccine deployment structures which would slow down vaccine delivery to the population as a whole, leaving some individuals unvaccinated for longer.
Q: How do I book a vaccine?
You can only book a vaccine if you have received a letter inviting you to book your vaccination appointments, then you can use the following service. If you do not have access to the internet, then call 119 if you’re in England, Wales or Northern Ireland or 0300 303 2713 if you’re in Scotland. Lines are open 7am to11pm.
The Government are introducing a National Booking Service to helping make the process of booking and accessing an appointment easier for those offered a vaccine in the coming days, weeks and months ahead.
Q: How will older people be contacted about the vaccine?
Last week saw the vaccine roll out gathering pace, which is exciting news for those receiving the vaccine and provides some much-needed positive news for the rest of us. Local Age UKs have reported that they are receiving calls from older people who are worried about how they will receive their vaccine and whether they have to travel to a mass vaccine centre which may be challenging for some of them.
Age UK have written a useful blog on what communications older people should expect to receive from the NHS, what choices they have on where to receive their vaccine and how to spot a scam. The blog includes:
- Which vaccine will I get?
- Where will I get the vaccine?
- How will I be contacted?
- Things to watch out for
With misinformation in the public domain, it is important that older people can find information from accessible and trustworthy sources. Age UK have a useful coronavirus guidance hub which includes frequently asked questions about the vaccine.
Q: What about the new variant and its effects on elderly people?
News of the new coronavirus variant is understandably very worrying. To help older people understand what this all means and how it’s affecting the vaccine roll out Age UK have produced a helpful blog to answer some basic questions.
Q: How will those who are housebound be vaccinated?
Housebound vaccinations have now started and are being delivered via the GP lead vaccination services. Residents will be contacted prior to a clinician attending their home. Residents are advised not to let any unauthorised persons into their home without prior knowledge.
Q: How effective is the vaccine?
- A single dose of the vaccine can reduce household transmission of the virus by up to half.
- Both vaccines reduce the likelihood of serious illness by 60 per cent after one dose.
- They reduce the likelihood of hospitalisation by 80 per cent after one dose in the over 80s too.
- And PHE estimate this vaccine programme has prevented over 10,000 deaths up to the end of March.